Novel Drug Reconstitution Systems Market, 2021-2030 by Roots Analysis
Novel Drug Reconstitution Systems Market, 2021-2030 by Roots Analysis
LONDON, ENGLAND, UNITED KINGDOM, October 18, 2021 /EINPresswire.com/ -- Roots Analysis has announced the addition of “Novel Drug Reconstitution Systems Market, 2021-2030” report to its list of offerings.
To order this 320+ page report, which features 230 figures and 248 tables, please visit https://www.rootsanalysis.com/reports/drug-reconstitution-systems-market.html
Key Inclusions
A detailed assessment of the current market landscape of novel drug reconstitution systems, providing information on the type of device (prefilled syringe, cartridge, infusion bags), type of chamber (dual chamber, multi chamber), physical state of drug (lyophilized, liquid), container fabrication material (glass, plastic), device usability (single use, multi-use), and volume of container. In addition, the chapter includes details related to novel drug reconstitution system manufacturers, along with information on their year of establishment, company size, location of headquarters and key players (in terms of number of products manufactured).
A detailed landscape of the reconstitution devices and systems featuring information on type of container or device, volume of primary container, physical state of drug, device usability and provision for self-administration. In addition, the chapter includes details related to the manufacturers, along with information on their year of establishment, company size and location of headquarters.
Elaborate profiles of prominent players engaged in this domain. Each profile includes a brief overview of the company, details related to its financial information (if available), information on product portfolio, recent developments and an informed future outlook.
A detailed analysis on the trends in packaging of over 350 drug products (including both biologics and small molecule drugs) that were approved by the FDA between 2014 and H1 2021, featuring an assessment of the packaging requirements of various container-closure systems based on several parameters, such as year of approval of drug, type of molecule (small molecule, biologic), type of biologic (allogeneic cell therapy, autologous cell therapy, fusion proteins, hormones, interferons, monoclonal antibodies, recombinant enzymes, recombinant protein and viral cell therapy), type of primary packaging container used (vials, pouches / packets, bottles, IV / sealed bags, prefilled syringes / pen , tubes, cartridge, blister packaging, others), type of packaging material(s) used for manufacturing primary container, type of closure used (cap / needle shield, seal, plunger, stopper and others), type of packaging material(s) used for manufacturing closures, dosage form, route of administration, holding temperature. In addition, the chapter provides information on the developers of the aforementioned drugs and an analysis based on year of establishment, company size, location of headquarters and leading drug developers (in terms of number of drugs approved).
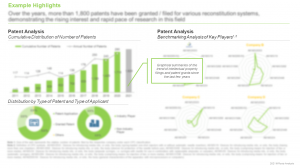
An insightful analysis of the patents filed / granted for novel drug reconstitution systems, since 2011, taking into consideration various relevant parameters, such as type of patent, publication year, geographical location, CPC symbols, emerging focus areas, leading players (in terms of number of patents granted / filed in the given time period), patent characteristics and geography. In addition, the chapter includes a detailed patent benchmarking and an insightful valuation analysis.
A competitiveness analysis of novel drug reconstitution system manufacturers based on various relevant parameters, such as supplier power (in terms of experience / expertise of the manufacturer) and key product specifications (number of systems, type of systems, type of drugs and number of chambers).
An in-depth analysis of recent events (summits / forums / conferences / annual meetings) that were organized for stakeholders in this domain, highlighting the evolution of discussion topics related to novel drug reconstitution systems. The analysis also provides details on type of event, regional distribution, emerging agendas, popular organizers, active industry and non-industry players, and a schematic mapping of upcoming events.
A discussion on affiliated trends, key drivers and challenges, under a SWOT framework, featuring a Harvey ball analysis, highlighting the relative impact of each SWOT parameter on the overall novel drug reconstitution systems market.
An in-depth analysis to estimate the current and future demand for various novel drug reconstitution systems, including cartridges, infusion bags and prefilled syringes.
An elaborate discussion on emerging trends that are likely to have an impact on the future adoption of novel drug reconstitution systems. It presents a Harvey ball analysis, highlighting the relative effect of each trend on the adoption of novel drug reconstitution systems including dual chamber systems.
The report also features the likely distribution of the current and forecasted opportunity across important market segments, mentioned below:
Type of Container
Prefilled Syringe
Cartridge
Infusion Bag
Fabrication Material
Glass
Plastic
Physical State of Drug in Syringe and Cartridge
Liquid / Powder
Liquid / Liquid
Physical State of Drug in Infusion Bag
Liquid Mixture
Frozen Mixture
Volume of Container
<1 mL, 1-2.5 mL, 2.5-5 mL, >5 mL for prefilled syringe and cartridge
250 mL, 250-500 mL, 500-1,000 mL, >1,000 mL for infusion bag
Key Geographical Regions
North America
Europe
Asia-Pacific
Latin America
Middle East and North Africa
To request sample pages, please visit https://www.rootsanalysis.com/reports/drug-reconstitution-systems-market.html
Key Questions Answered
Who are the key players engaged in the development of novel drug reconstitution systems?
What is the relative competitiveness of different novel drug reconstitution system manufacturers?
What is the packaging trend in terms of container and closure for the drugs approved since 2014?
Who are the leading players focused on the development of lyophilized drugs?
What is the focus area of various conferences related to novel drug reconstitution systems?
How has the intellectual property landscape of novel drug reconstitution systems evolved over the years?
What are the emerging trends related to pharmaceutical packaging?
What are the key agenda items being discussed in various global events / conferences related to novel drug reconstitution systems?
How is the current and future market opportunity likely to be distributed across key market segments?
You may also be interested in the following titles:
1. Novel Ocular Drug Delivery Devices Market, 2021-2030
2. Pre-Sterilized / Ready-to-Use Primary Packaging Market, 2021-2030
3. Medical Device Batteries Market, 2020-2030
Contact Us
Ben Johnson
+1 (415) 800 3415
Ben.johnson@rootsanalysis.com
Gaurav Chaudhary
Roots Analysis
+1 415-800-3415
email us here
Visit us on social media:
Facebook
Twitter
LinkedIn
EIN Presswire does not exercise editorial control over third-party content provided, uploaded, published, or distributed by users of EIN Presswire. We are a distributor, not a publisher, of 3rd party content. Such content may contain the views, opinions, statements, offers, and other material of the respective users, suppliers, participants, or authors.